- Home
- Products
- Pathway
- Support
- Contact Us

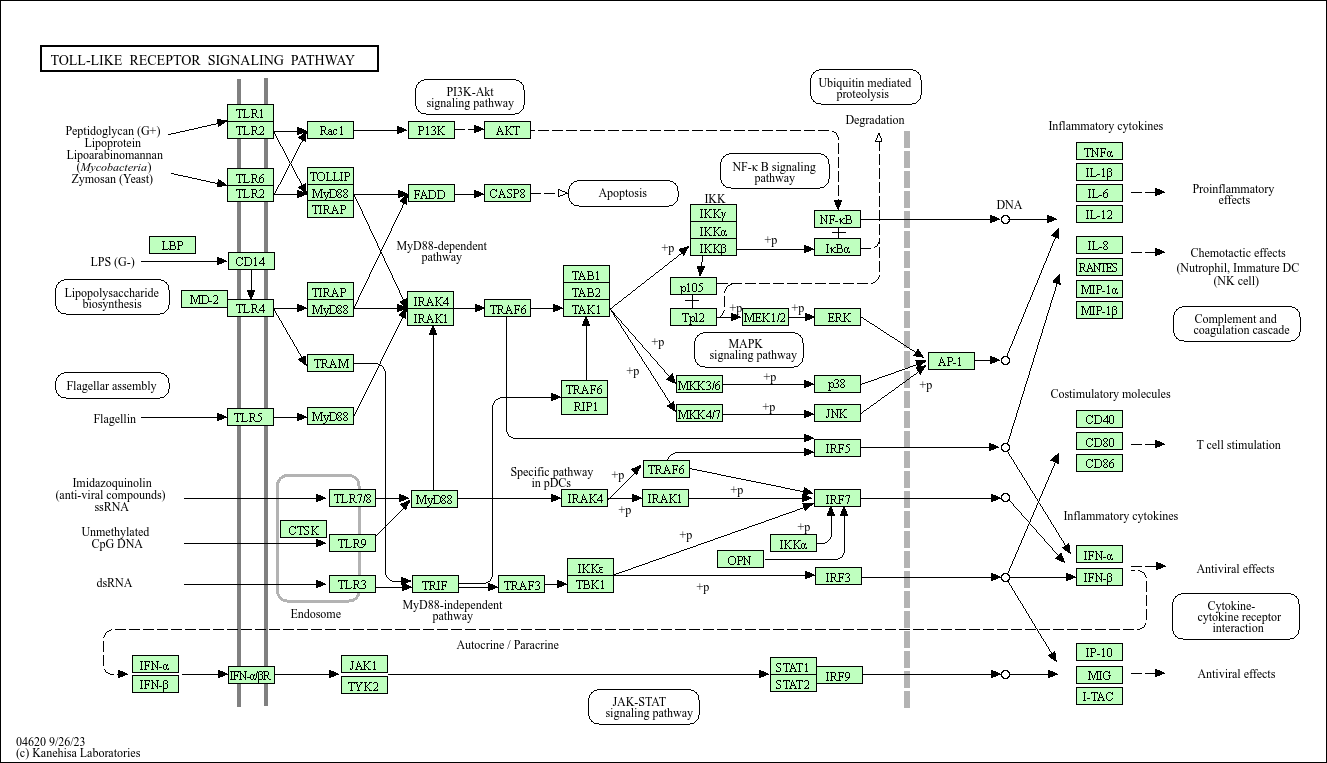
Toll-like receptor signaling pathway
Core of basic research: As a "sentinel" pathway of innate immunity, it deciphers the mechanism by which TLR family receptors recognize pathogen-associated molecular patterns (PAMPs) to activate inflammatory responses. TLR family members have distinct expression localizations and ligand specificities: TLR1/2/4/5/6 are expressed on the cell membrane, recognizing bacterial lipoproteins, lipopolysaccharides (LPS), flagellin, etc.; TLR3/7/8/9 are expressed in endosomes, recognizing viral double-stranded RNA, single-stranded RNA, and bacterial DNA (CpG motifs). TLRs dimerize upon ligand binding, recruiting signal adaptors such as MyD88 (except TLR3) or TRIF (TLR3, TLR4), activating kinases like IRAK4 and TAK1, which in turn activate the NF-κB and MAPK pathways to secrete inflammatory factors (TNF-α, IL-6) and type I interferons. Research focuses on TLR ligand recognition specificity, differences between MyD88-dependent and TRIF-dependent pathways, negative regulatory mechanisms (e.g., SOCS1, TOLLIP), and associations between excessive TLR activation and chronic inflammation or autoimmune diseases.
Core key proteins: TLR1-10 (receptor family), MyD88, TRIF (signal adaptors), IRAK4, TAK1 (kinases), NF-κB (p65/p50), MAPK (p38/ERK/JNK), IFN-α/β, TNF-α, IL-6, TOLLIP (negative regulator), MD2 (TLR4 auxiliary protein), CD14 (LPS-binding protein).
Core key proteins: TLR1-10 (receptor family), MyD88, TRIF (signal adaptors), IRAK4, TAK1 (kinases), NF-κB (p65/p50), MAPK (p38/ERK/JNK), IFN-α/β, TNF-α, IL-6, TOLLIP (negative regulator), MD2 (TLR4 auxiliary protein), CD14 (LPS-binding protein).
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















