- Home
- Products
- Pathway
- Support
- Contact Us

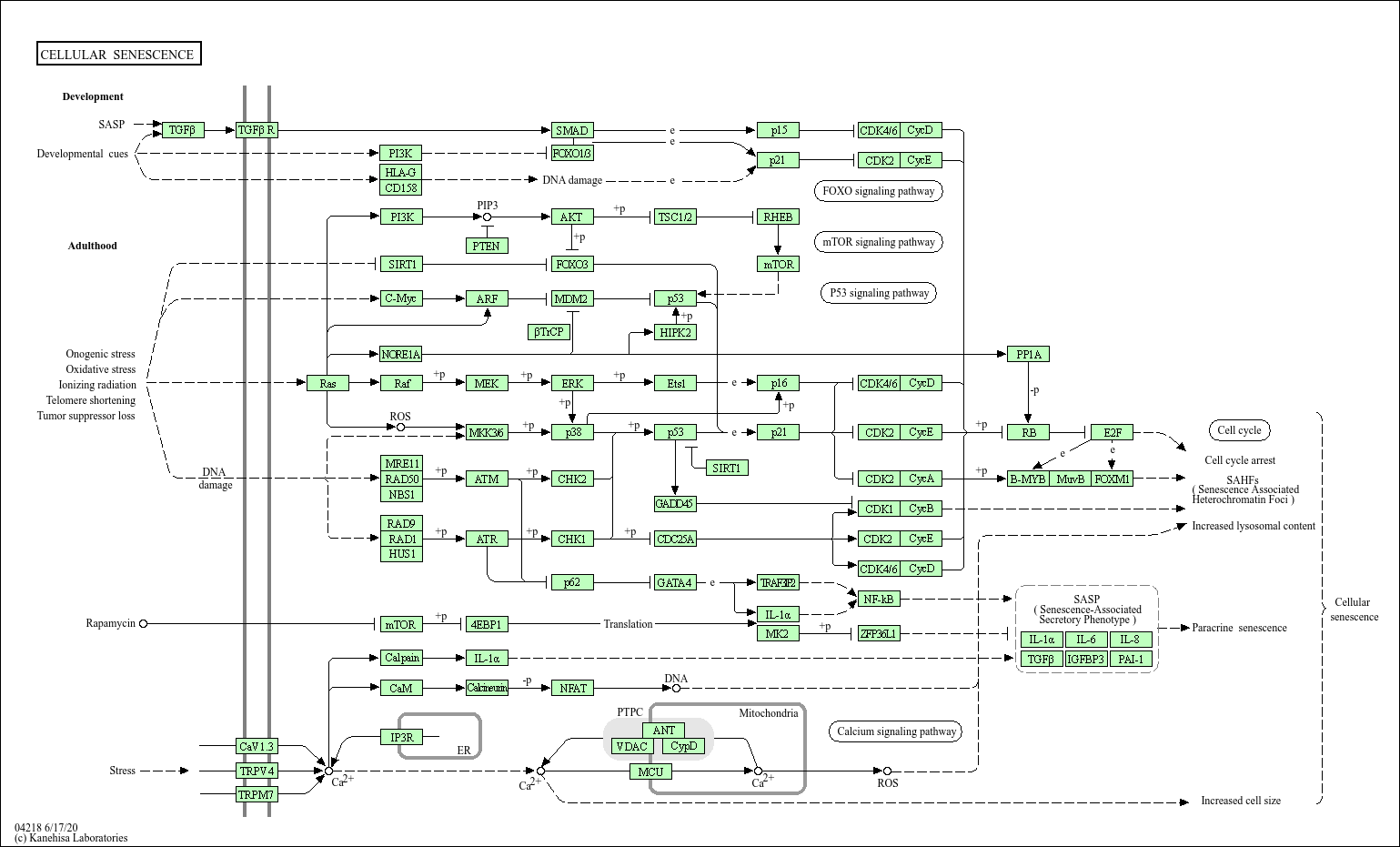
Cellular senescence
Core of basic research: Deciphers the molecular mechanism by which cells enter an irreversible proliferative arrest state under stress stimulation, critical for inhibiting tumorigenesis and regulating aging processes. Core triggering factors include DNA damage, telomere shortening, oxidative stress, and oncogene activation, ultimately initiating senescence via two core pathways: The p53-p21 pathway (DNA damage activates p53, inducing p21 expression to inhibit Cyclin-CDK complexes, leading to cell cycle arrest); the p16INK4a-Rb pathway (upregulated p16INK4a inhibits CDK4/6, maintaining Rb in a hypophosphorylated inhibitory state to block E2F-mediated proliferative signals). Senescent cells secrete Senescence-Associated Secretory Phenotype (SASP) factors (IL-6, TNF-α, MMPs, etc.), affecting surrounding cell functions and the tissue microenvironment. Research focuses include telomere shortening-mediated senescence triggering mechanisms, associations between oxidative stress and DNA damage, regulatory networks of SASP factors, senescent cell clearance mechanisms, and the association of pathway abnormalities with tumors (senescence escape) and age-related diseases (SASP-mediated chronic inflammation).
Core key proteins: p53, p21/Cip1, p16INK4a, Rb, telomere-related proteins (TERT, TRF1/TRF2, maintaining telomere length), DNA damage response proteins (ATM/ATR, γ-H2AX), SASP-related factors (IL-6, TNF-α, IL-8, MMP3/9), p38 MAPK (regulating SASP secretion), FOXO transcription factors (regulating antioxidant and senescence-related genes), β-galactosidase (senescent cell marker).
Core key proteins: p53, p21/Cip1, p16INK4a, Rb, telomere-related proteins (TERT, TRF1/TRF2, maintaining telomere length), DNA damage response proteins (ATM/ATR, γ-H2AX), SASP-related factors (IL-6, TNF-α, IL-8, MMP3/9), p38 MAPK (regulating SASP secretion), FOXO transcription factors (regulating antioxidant and senescence-related genes), β-galactosidase (senescent cell marker).
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















