
I. Discovery Origin and Scientific Definition
Nanobodies (Nb), also known as Single-Domain Antibodies (sdAb), have their core functional unit as the Variable Domain of Heavy Chain of Heavy-Chain Antibody (VHH). In 1989, Belgian scientists first discovered natural heavy-chain antibodies without light chains in the blood of camelids. In 1993, the Hamers-Casterman team clarified their properties in the journal Nature — the variable domain of the heavy chain can exist independently of the constant domain while maintaining antigen-binding activity. It is named "Nanobody" due to its crystal size of only 2.5nm×4nm and molecular weight of 12-15 kDa (approximately 1/10 that of traditional IgG antibodies), and it also exhibits superior tolerance to extreme environments.
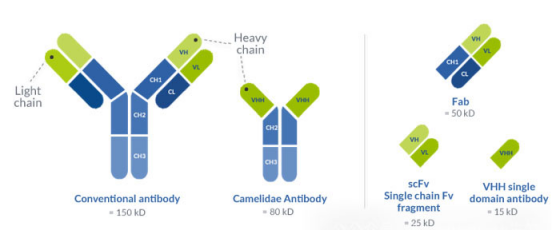
II. Core Structure Analysis and Key Characteristics
(A) Unique Molecular Structure
Naturally lacking light chains, it consists solely of the variable domain of heavy chain (VHH) as its functional core, with three structural features:
1 Sequence Feature: Comprises 3 hypervariable regions (CDR1/CDR2/CDR3) and 4 framework regions (FR1-FR4). Four aliphatic amino acids in the FR2 region are replaced by hydrophilic residues, significantly improving water solubility and preventing aggregation.
2 Binding Site Characteristic: The CDR3 region is notably extended (16-18 amino acids) and can form convex, grooved, or pocket-like conformations, compensating for the loss of binding affinity caused by the absence of light chains.
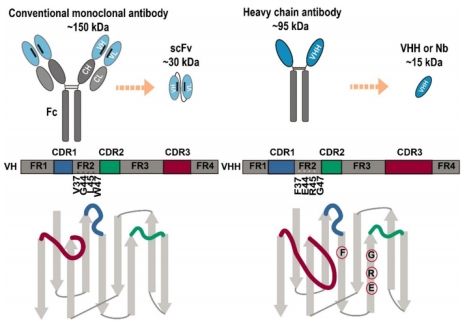
3 Stability Foundation: Contains additional interloop disulfide bonds internally, which maintain conformational stability and enhance stress resistance.
(B)Nine Core Advantages
Biological Characteristic | Specific Performance | Technical Value |
Molecular property | With a molecular weight of 12-15 kDa, it features strong tissue penetration, capable of crossing the blood-brain barrier and penetrating the tumor microenvironment. | Enables targeted delivery to deep lesions and enhances drug delivery efficiency. |
Immunological safety | It shares 90% structural homology with human VH and exhibits extremely low immunogenicity post humanization. | Mitigates the risk of immune rejection in clinical applications. |
Specificity | It boasts high specificity for recognizing native protein conformations; its long, flexible CDR3 region enables binding to cryptic antigen epitopes inaccessible to traditional antibodies. | Expands the spectrum of targetable antigens, with particular suitability for conformational antigens. |
Affinity | Its dissociation constant (Kd) ranges from nanomolar to picomolar levels, with binding specificity comparable to or even superior to that of traditional antibodies. | Ensures precision in diagnosis and therapy. |
Physicochemical stability | It tolerates pH 2-11, 85°C high temperature, proteases, and organic solvents, and can refold to regain activity after denaturation. | It supports room-temperature storage and transportation, and is compatible with multiple administration routes such as oral and aerosol delivery. |
Preparation convenience | It enables high-efficiency expression in prokaryotic/eukaryotic systems (e.g., E. coli, yeast), with an expression level significantly higher than that of traditional antibody fragments. | Reduces production costs and shortens manufacturing cycles. |
Engineering flexibility | It facilitates easy humanization and enables the construction of bispecific, multivalent antibodies or fusion proteins. | Addresses diverse application requirements. |
Batch stability | Recombinant expression via genetic engineering ensures high consistency in sequence and activity across batches. | Guarantees stability for both clinical applications and industrial manufacturing. |
Screening efficiency | Rapid screening is achievable via phage display libraries, with a cycle of only several weeks. | Accelerates target validation and drug R&D processes. |
III. Mainstream Preparation Technologies and Industrialization Support
(A) Core Preparation Process
Currently, phage display technology is the core, with a typical process including:
Antigen Immunization: Immunize hosts such as camels and alpacas with the target antigen, and collect blood after 4-5 immunizations.
Library Construction: Extract lymphocyte RNA, reverse transcribe it into cDNA, amplify the VHH gene, and construct a phage display library.
Screening and Purification: Obtain high-affinity clones through multiple rounds of panning, followed by expression and purification in prokaryotic/eukaryotic systems.
Activity Verification: Verify binding activity and specificity using techniques such as ELISA and SPR.
(B) Industrialization Technology Platform
A fully human protein phage display nanobody library has been established, forming a full-chain technical system covering "antigen synthesis → library screening → sequencing identification → recombinant expression". Currently, nanobody development for over 200 targets has been completed, enabling rapid acquisition of high-quality target molecules.
IV. Multi-Field Application Scenarios and Latest Progress
(A) Disease Treatment Field
Launched Drugs: In 2018, Cablivi (caplacizumab), the world’s first nanobody drug, was approved for the treatment of thrombotic thrombocytopenic purpura (TTP).
Tumor Treatment: Nanobody-based CAR-T therapies targeting EGFR, BCMA and other targets have entered Phase Ⅲ clinical trials, with complete remission lasting over 15 months in some patients.
Neurological Diseases: In 2025, an international team developed glutamate receptor-activating nanobodies that can improve cognitive impairment in schizophrenia via peripheral injection, with efficacy lasting over one week.
Infectious Diseases: Anti-SARS-CoV-2 nanobodies show good stability after lyophilization and aerosolization, and have been used in the research and development of nasal sprays.
(B) Diagnostic Detection Field
Clinical Diagnosis: Integrated into colloidal gold test strips and electrochemiluminescence platforms, they are used for rapid and accurate detection of tumor markers (e.g., CEA, CA125) and pathogens (e.g., COVID-19 virus, influenza virus).
Research Tools: Compatible with techniques such as FCM, IP, WB, and IHC/IF, they enable high-sensitivity detection of low-abundance proteins, facilitating research on protein interactions and metabolic regulation.
(C) Featured Application Directions
In Vivo Imaging: After labeling with fluorescent dyes or radioisotopes, they enable multi-modal imaging (optical/MRI/CT) of tumors and pathogens, with a tumor-to-background ratio significantly higher than that of traditional antibodies.
Targeted Radiotherapy: Acting as radioisotope carriers, they are accurately delivered to tumor cells, reducing damage to normal tissues.
Drug Delivery: Conjugated with chemotherapeutic drugs or toxins, they achieve precise release at lesion sites, lowering systemic toxic side effects.
Food Safety: Specific nanobodies against pesticide residues (e.g., fenitrothion, procymidone) and biological toxins have been developed for rapid detection.
V. Current Development Status and Future Outlook
Globally, over 40 nanobody drug candidates are currently in the R&D pipeline, covering areas such as cancer, autoimmune diseases, and neurological diseases. Chinese enterprises have made rapid progress in PD-L1 nanobodies, CAR-T therapies and other directions, with some products simultaneously conducting multi-center clinical trials in China, the US, and Japan. With breakthroughs in the construction of billion-scale libraries and high-throughput screening technologies, as well as in-depth R&D of multispecific nanobodies and intracellular targeting nanobodies, the application potential of nanobodies—hailed as "next-generation biotech missiles"—will be further unleashed. They are expected to achieve disruptive breakthroughs in fields such as precision medicine and point-of-care testing.
Related Promotional Journal Downloads

30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us



















