- Home
- Products
- Pathway
- Support
- Contact Us

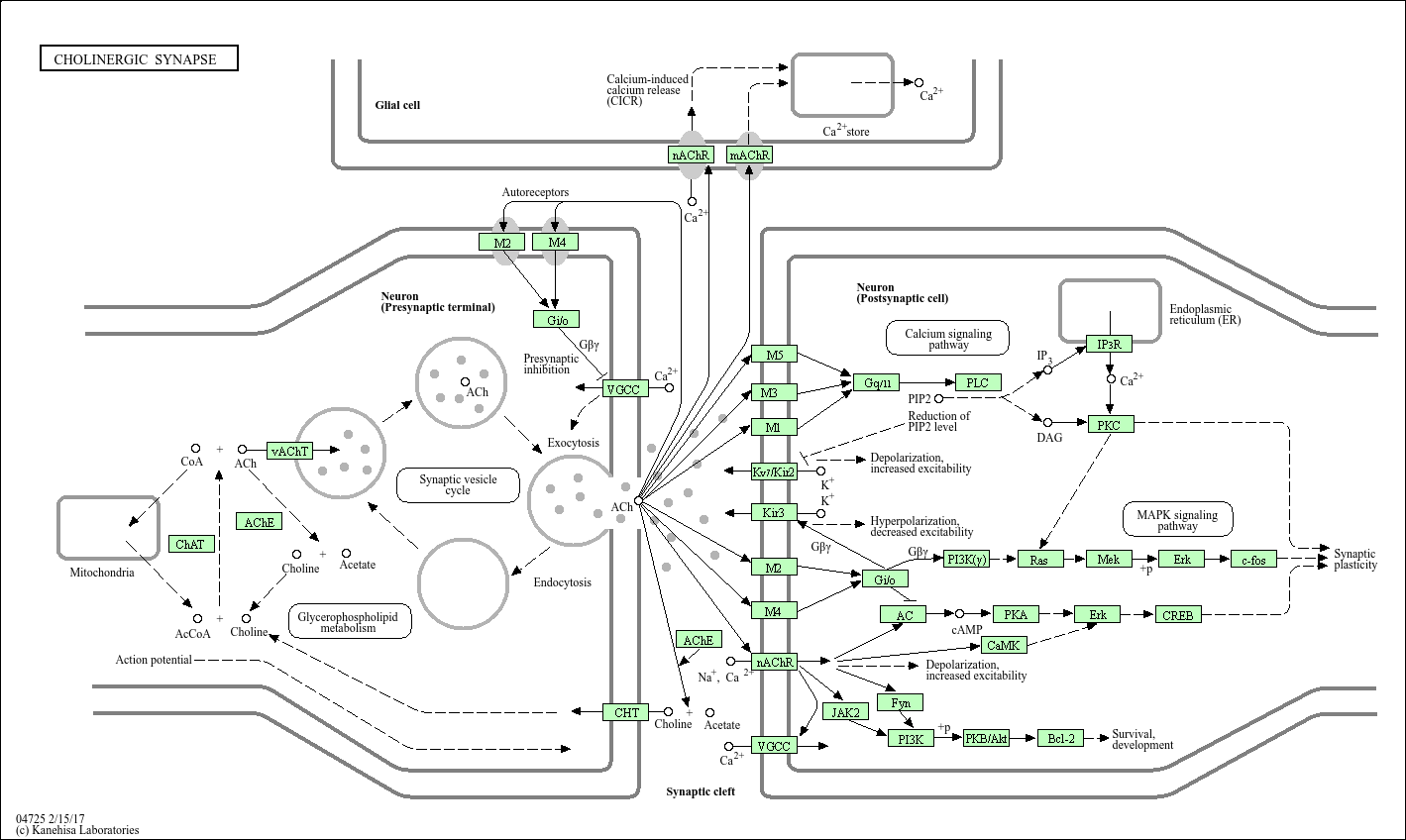
Cholinergic synapse
Core of basic research: Deciphers the synaptic transmission mechanism of acetylcholine (ACh) as a neurotransmitter, widely involved in motor control, cognitive function, autonomic regulation, and learning/memory. When presynaptic neurons are excited, action potentials induce calcium influx, triggering exocytosis of ACh-containing synaptic vesicles with the presynaptic membrane to release ACh into the synaptic cleft. ACh binds to cholinergic receptors on the postsynaptic membrane, mediating two types of effects: nicotinic receptors (nAChR, ion channel-type) activate sodium influx to induce rapid postsynaptic depolarization, mediating fast neural conduction (e.g., neuromuscular junction transmission); muscarinic receptors (mAChR, G protein-coupled) regulate downstream signals via Gq/Gi proteins (e.g., PLC-γ pathway or inhibiting AC activity), mediating slow and sustained physiological effects (e.g., glandular secretion, smooth muscle contraction). ACh in the synaptic cleft is hydrolyzed into choline and acetic acid by acetylcholinesterase (AChE) to terminate signal transmission. Research focuses on the regulatory mechanism of synaptic vesicle exocytosis, subtype-specific functions of cholinergic receptors, regulation of AChE hydrolysis efficiency, and pathway abnormalities in neurodegenerative diseases (Alzheimer’s disease, reduced ACh secretion) and myasthenia gravis (nAChR antibody blockage).
Core key proteins: Acetylcholine (ACh), nicotinic cholinergic receptors (nAChR, α/β/γ/δ subunits), muscarinic cholinergic receptors (mAChR, M1-M5 subtypes), acetylcholinesterase (AChE), choline acetyltransferase (ChAT, key ACh synthesis enzyme), Synaptotagmin (calcium sensor mediating exocytosis), Gq/Gi proteins (mAChR-coupled proteins), PLC-γ (phospholipase C-γ), IP3R (endoplasmic reticulum calcium channel), potassium channels (regulated by mAChR), sodium channels (nAChR-associated ion channels), presynaptic/postsynaptic membranes, neuromuscular junction.
Core key proteins: Acetylcholine (ACh), nicotinic cholinergic receptors (nAChR, α/β/γ/δ subunits), muscarinic cholinergic receptors (mAChR, M1-M5 subtypes), acetylcholinesterase (AChE), choline acetyltransferase (ChAT, key ACh synthesis enzyme), Synaptotagmin (calcium sensor mediating exocytosis), Gq/Gi proteins (mAChR-coupled proteins), PLC-γ (phospholipase C-γ), IP3R (endoplasmic reticulum calcium channel), potassium channels (regulated by mAChR), sodium channels (nAChR-associated ion channels), presynaptic/postsynaptic membranes, neuromuscular junction.
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















