- Home
- Products
- Pathway
- Support
- Contact Us

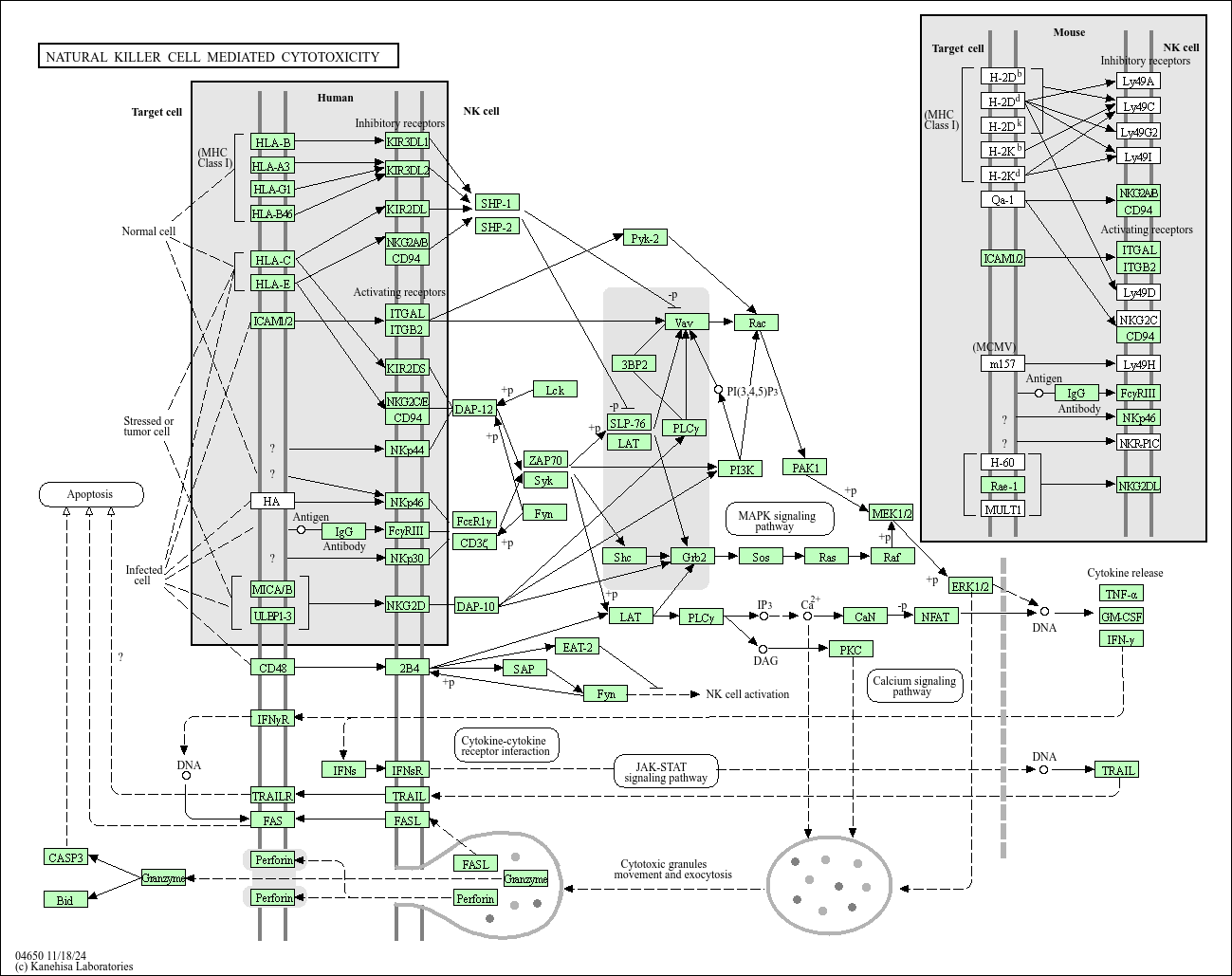
Natural killer cell mediated cytotoxicity
Core of basic research: It explores the "dual-signal" regulatory mechanism by which NK cells recognize and kill abnormal cells (tumor cells, virus-infected cells), relying on the balance between activating and inhibitory receptors. Activating receptors (NKG2D, NKp30) recognize stress molecules (MICA/B, ULBP) on the target cell surface to transmit killing signals; inhibitory receptors (KIR family, CD94-NKG2A) recognize normally expressed MHC-I molecules on the target cell surface to transmit inhibitory signals. When MHC-I molecule expression is downregulated (e.g., tumor cell immune escape) or stress molecules are highly expressed on target cells, activating signals predominate. NK cells kill target cells in two ways: ① Releasing Perforin to form membrane pores, allowing Granzyme B to enter target cells and initiate apoptosis; ② Initiating the extrinsic apoptotic pathway via the Fas-FasL or TNF-α-TNFR1 pathway. Research focuses include the specific mechanism of receptor recognition, regulation of killer granule release, interactions between NK cells and DC cells (enhancing killing activity), and inhibitory mechanisms of NK cell function in the tumor microenvironment.
Core key proteins: NK cell receptors (NKG2D, KIR family, NKp30/NKp46), Perforin, Granzyme B, Fas/FasL, TNF-α/TNFR1, caspase-3/8 (apoptosis execution enzymes), MICA/B (stress molecules), IFN-γ (enhances killing activity), Eomes/T-bet (NK cell function regulatory transcription factors).
Core key proteins: NK cell receptors (NKG2D, KIR family, NKp30/NKp46), Perforin, Granzyme B, Fas/FasL, TNF-α/TNFR1, caspase-3/8 (apoptosis execution enzymes), MICA/B (stress molecules), IFN-γ (enhances killing activity), Eomes/T-bet (NK cell function regulatory transcription factors).
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















