- Home
- Products
- Pathway
- Support
- Contact Us

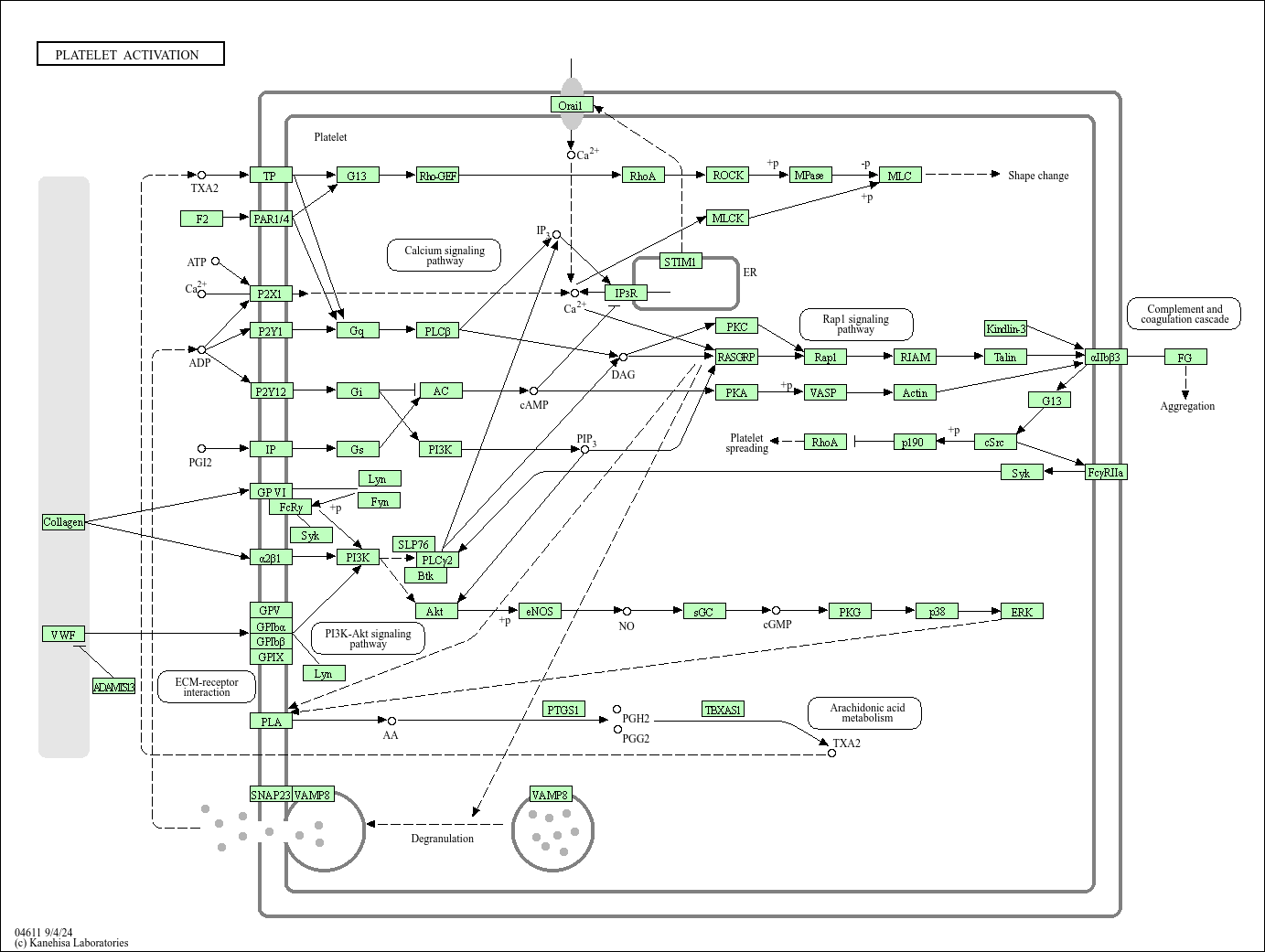
Platelet activation
Core of basic research: Deciphers the three-step molecular mechanism ("adhesion-aggregation-degranulation") of platelet-initiated hemostasis and thrombosis after vascular injury. Exposed subendothelial collagen post-injury binds platelets via GPVI receptors and GPIb-IX-V complexes (with vWF), completing adhesion. Adhesion triggers platelet activation, increasing intracellular calcium concentration and inducing release of granular contents (e.g., ADP, thromboxane A2/TXA2). These released substances act as secondary activation signals, binding to platelet surface P2Y12 (ADP receptor) and TP (TXA2 receptor) to amplify activation, inducing conformational changes and activation of GPⅡb/Ⅲa receptors. Activated GPⅡb/Ⅲa binds fibrinogen, mediating platelet cross-linking and aggregation to form white thrombi. Research focuses on the signal transduction cascade of platelet activation (PLC-γ2, PI3K-Akt pathways), conformational regulation of GPⅡb/Ⅲa activation, targets of antiplatelet drugs (aspirin, clopidogrel), and molecular triggers of excessive platelet activation in thrombotic diseases (myocardial infarction, cerebral infarction).
Core key proteins: Platelets, GPVI (collagen receptor), GPIb-IX-V (vWF receptor), GPⅡb/Ⅲa (fibrinogen receptor), vWF (von Willebrand factor), ADP (secondary activation signal), TXA2 (thromboxane A2), P2Y12 (ADP receptor), TP (TXA2 receptor), PLC-γ2 (key signal pathway enzyme), PI3K/Akt (core signal pathway molecules), PKC (calcium signal downstream kinase), CaM (calmodulin), TXS (thromboxane synthase), fibrinogen (platelet aggregation cross-linking molecule).
Core key proteins: Platelets, GPVI (collagen receptor), GPIb-IX-V (vWF receptor), GPⅡb/Ⅲa (fibrinogen receptor), vWF (von Willebrand factor), ADP (secondary activation signal), TXA2 (thromboxane A2), P2Y12 (ADP receptor), TP (TXA2 receptor), PLC-γ2 (key signal pathway enzyme), PI3K/Akt (core signal pathway molecules), PKC (calcium signal downstream kinase), CaM (calmodulin), TXS (thromboxane synthase), fibrinogen (platelet aggregation cross-linking molecule).
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















