- Home
- Products
- Pathway
- Support
- Contact Us

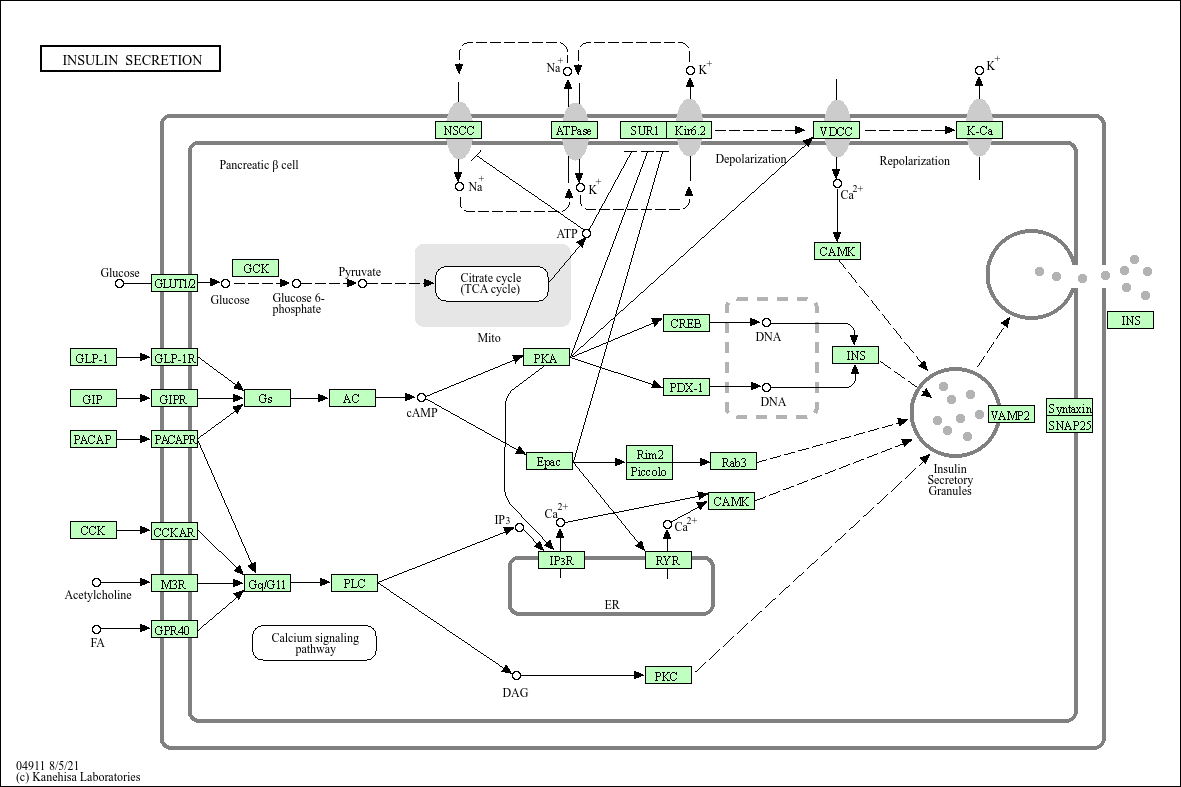
Insulin secretion
Core of basic research: A core link in regulating systemic glucose homeostasis, focusing on the molecular mechanisms by which pancreatic β cells secrete insulin under the regulation of glucose and other signals. The core is a three-level regulation: "glucose sensing - signal transduction - insulin granule exocytosis". Glucose enters β cells via GLUT2, generating ATP through glycolysis and the tricarboxylic acid cycle, which closes KATP channels and depolarizes the cell membrane. Depolarization activates voltage-dependent L-type calcium channels (VDCC), and calcium influx triggers the fusion (exocytosis) of insulin granules with the cell membrane, releasing insulin into the bloodstream. Additionally, incretins (e.g., GLP-1), amino acids, and neurotransmitters act as synergistic signals: GLP-1 binds to its receptor to activate the cAMP-PKA pathway, enhancing calcium influx and insulin granule exocytosis efficiency; amino acids assist in regulating secretion via metabolism or direct activation of ion channels. Research focuses on the specific mechanism of glucose sensing, the regulatory logic of KATP channels, the molecular switch of exocytosis, and pathway abnormalities associated with type 1 diabetes (insufficient secretion due to β cell destruction) and type 2 diabetes (β cell secretion dysfunction).
Core key proteins: Insulin, GLUT2 (glucose transporter 2), GK (glucokinase), KATP channel (composed of Kir6.2 and SUR1 subunits), VDCC (mainly Cav1.2/1.3), Ca²+ (calcium ion), Synaptotagmin-7 (calcium sensor mediating exocytosis), SNARE complex (Syntaxin-1A, VAMP2, SNAP-25), GLP-1 (glucagon-like peptide-1), GLP-1R (GLP-1 receptor), PKA (protein kinase A), Epac2 (exchange protein), pancreatic β cells.
Core key proteins: Insulin, GLUT2 (glucose transporter 2), GK (glucokinase), KATP channel (composed of Kir6.2 and SUR1 subunits), VDCC (mainly Cav1.2/1.3), Ca²+ (calcium ion), Synaptotagmin-7 (calcium sensor mediating exocytosis), SNARE complex (Syntaxin-1A, VAMP2, SNAP-25), GLP-1 (glucagon-like peptide-1), GLP-1R (GLP-1 receptor), PKA (protein kinase A), Epac2 (exchange protein), pancreatic β cells.
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















