- Home
- Products
- Pathway
- Support
- Contact Us

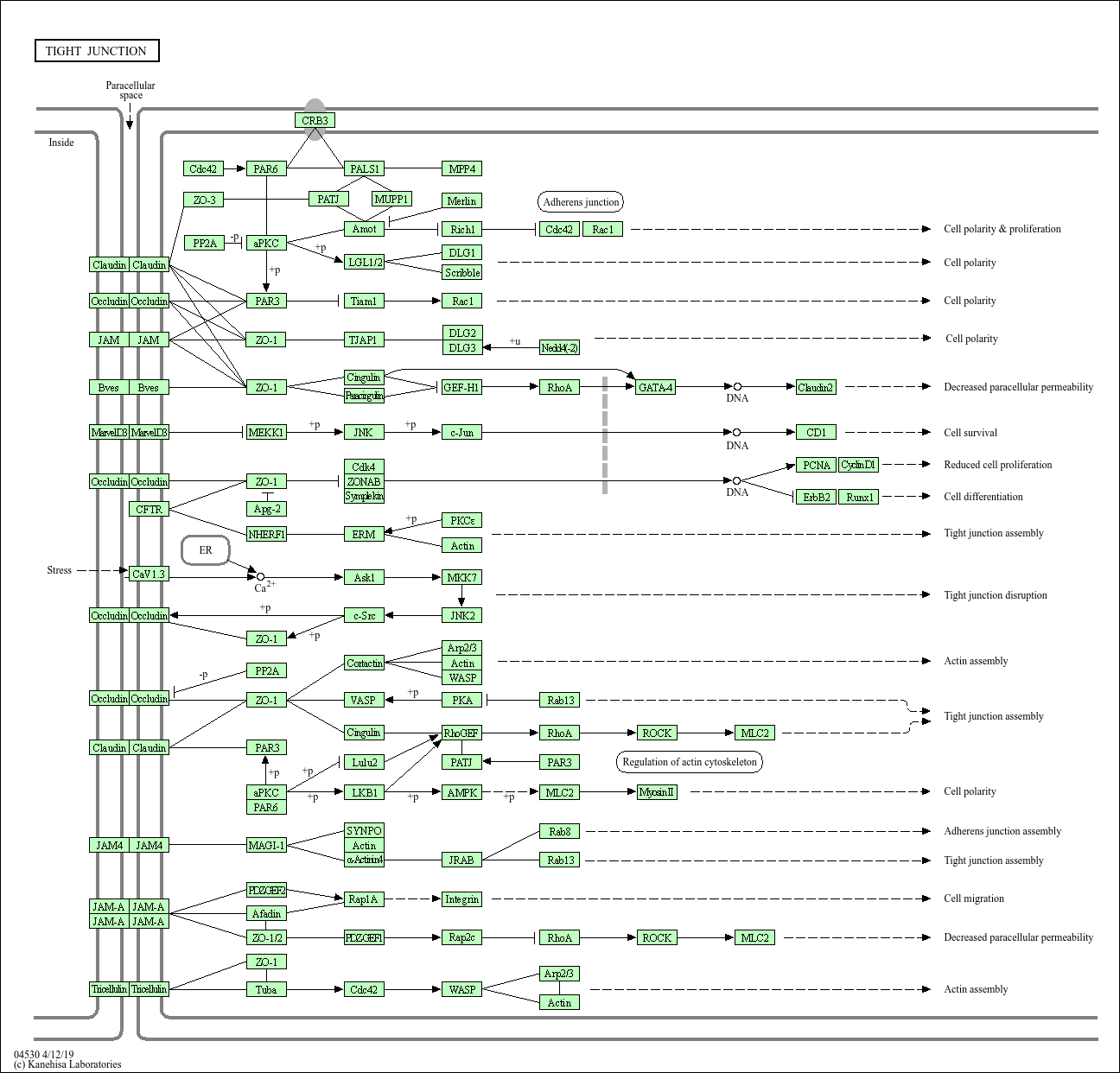
Tight junction
Core of basic research: Deciphers the molecular mechanism by which tight junctions seal intercellular gaps in epithelial/endothelial cells and maintain barrier function, critical for regulating trans-epithelial/endothelial transport. The core structure is a transmembrane protein-cytoplasmic scaffold protein complex: Transmembrane proteins (Occludin, Claudin, JAM) form intercellular sealing belts; their intracellular domains bind ZO family proteins (ZO-1, ZO-2, ZO-3), further linking to the actin cytoskeleton to prevent unselective small molecule penetration. This pathway not only forms a physical barrier but also participates in cell polarity establishment and regulates cell proliferation and differentiation. Research focuses include the tissue specificity of Claudin subtypes (e.g., Claudin-1 maintaining skin barrier), dynamic assembly and disassembly of tight junctions, associations between barrier function and signaling pathways, and the association of pathway abnormalities with inflammatory bowel disease (impaired intestinal barrier) and nephrotic syndrome (damaged glomerular barrier).
Core key proteins: Occludin, Claudin family (Claudin-1~27), Junctional Adhesion Molecules (JAM-A/B/C), ZO family proteins (ZO-1, ZO-2, ZO-3), Cingulin, Paracingulin, Actin, Myosin Light Chain Kinase (MLCK, regulating tight junction permeability), cell polarity proteins (Par3, Par6).
Core key proteins: Occludin, Claudin family (Claudin-1~27), Junctional Adhesion Molecules (JAM-A/B/C), ZO family proteins (ZO-1, ZO-2, ZO-3), Cingulin, Paracingulin, Actin, Myosin Light Chain Kinase (MLCK, regulating tight junction permeability), cell polarity proteins (Par3, Par6).
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















