- Home
- Products
- Pathway
- Support
- Contact Us

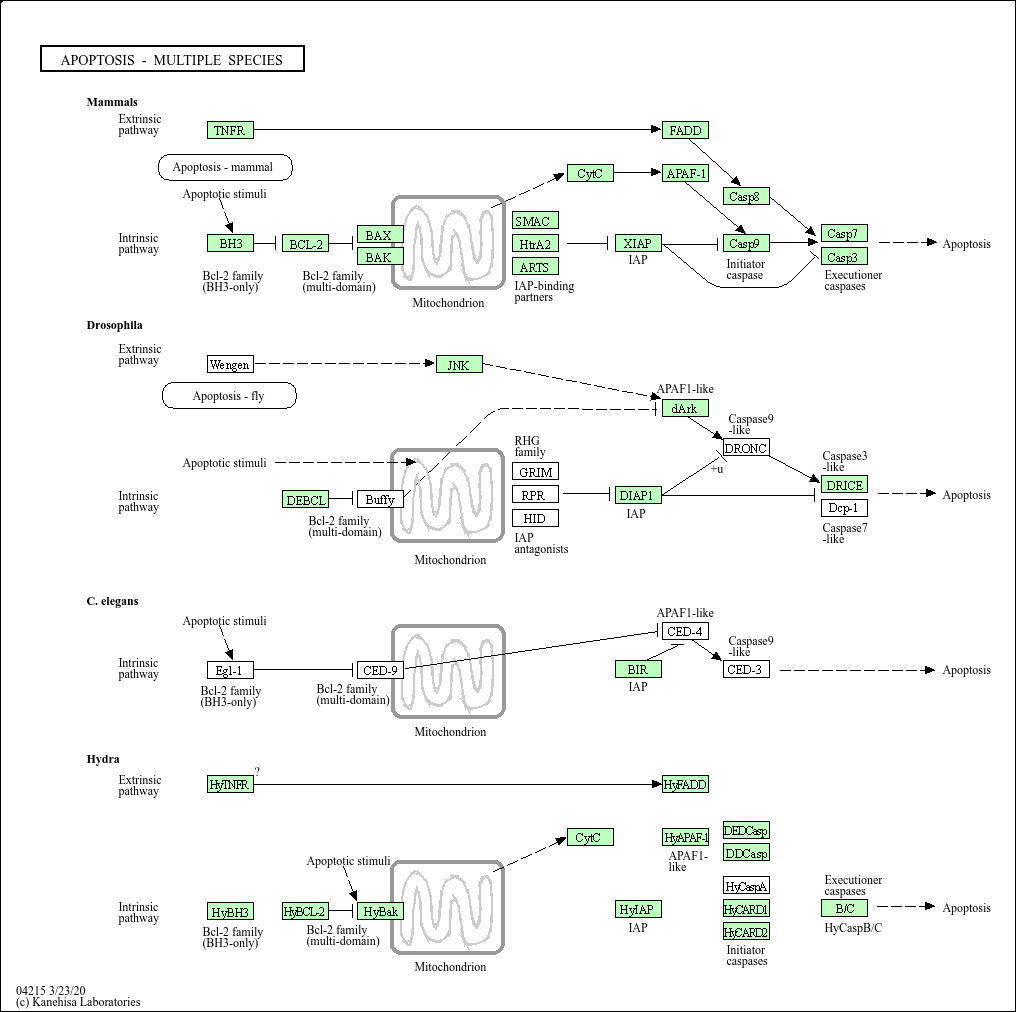
Apoptosis - multiple species
Core of basic research: Explores conserved apoptosis regulatory mechanisms from C. elegans and Drosophila to mammals, revealing the evolutionary conservation and species-specificity of apoptotic pathways. In C. elegans, apoptosis is regulated by Ced-3 (caspase homolog), Ced-4 (Apaf-1 homolog), and Ced-9 (Bcl-2 homolog); Ced-9 inhibits Ced-4-Ced-3 complex formation, and apoptotic signals relieve this inhibition to initiate death. In Drosophila, death receptors (Drsl) bind ligands (Eiger), activating Dronc (caspase-9 homolog) and Drice (caspase-3 homolog). In mammals, apoptotic pathways are conserved with lower organisms, with additional complex regulatory layers such as the p53 network and IAP family. Research focuses include the functional conservation of core apoptotic genes, the role of species-specific regulatory factors, evolutionary adaptation of apoptotic mechanisms, and the translational application of cross-species apoptotic mechanisms (e.g., C. elegans apoptosis models for anti-cancer drug screening).
Core key proteins:C. elegans: Ced-3 (caspase homolog), Ced-4 (Apaf-1 homolog), Ced-9 (Bcl-2 homolog), Ced-1/6/7 (apoptotic cell clearance-related);Drosophila: Dronc (initiator caspase), Drice (effector caspase), Eiger (TNF homolog), Drsl (death receptor homolog);Mammals: Caspase family, Bcl-2 family, p53, IAP family (conserved core proteins).
Core key proteins:C. elegans: Ced-3 (caspase homolog), Ced-4 (Apaf-1 homolog), Ced-9 (Bcl-2 homolog), Ced-1/6/7 (apoptotic cell clearance-related);Drosophila: Dronc (initiator caspase), Drice (effector caspase), Eiger (TNF homolog), Drsl (death receptor homolog);Mammals: Caspase family, Bcl-2 family, p53, IAP family (conserved core proteins).
Product list
-
{{item.title}}{{item.react}}{{item.applicat}}
Product list
Product name
Reactivity
Application
Related Resource Links
Related Promotional Journal Downloads

Explore Our Recommended Popular Products
More products
30,000+ high- quality products available online
Primary Antibodies, Secondary Antibodies, mIHC Kits, ELISA Kits, Proteins, Molecular Biology Products,Cell Lines,Reagents ...
Contact Us


















